Harnessing plasmons for green (photo)chemistry
Light is a powerful scalpel and carrier of information. If not for the diffraction-limit we would be able to focus light to nanometre length-scales, directly observe how molecules and atoms behave, and spectrally resolve their interactions. We would also be able to optically deliver just enough energy to exactly where it is needed for breaking molecular bonds and catalysing chemical reactions, eliminating the need for inefficient bulk-scale heating. Bypassing conventional optics, such nanoscale confinement of light does become possible using metals where incident light excites collective electron oscillations (plasmons) circumventing the diffraction limit.
Current work:
Plasmon Enabled Photocatalysis
By combining the powerful optical properties of plasmonic nanomaterials with catalytically active compounds new optical nanotechnologies can be developed that are capable of efficiently converting optical energy into chemical work. [1]
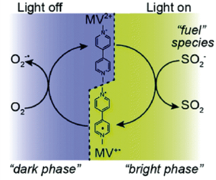
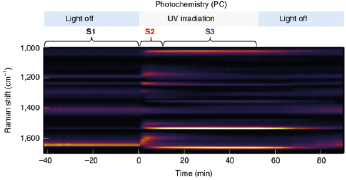
Optically Controlled Chemistry
By using plasmonic constructs local reaction conditions can be modified, eliminating e.g. the need for slow and inefficient bulk scale heating. This allows for rapid switching on and off chemical reactions using light. [2]
Key papers:
- Nanoparticle surfactants for kinetically-arrested photoactive assemblies…, Nature Nano (2021); DOI: 10.1038/s41565-021-00949-6
- Hot electron science in plasmonics and catalysis: what we argue about, Faraday Discussions (2019); DOI: 10.1039/C9FD00027E
Current people involved:
Bart deNijs, Ishaan Lohia, Christine Querebillo, Santiago Rodriguez Jimenez
Physics for Sustainable Chemistry group website: https://www.bartdenijs.com/people